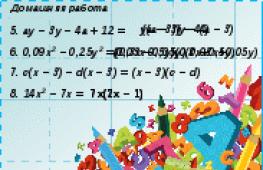The role of organic chemistry in human life message. Subject of organic chemistry
MOU "Secondary school r.p. Ozinki"
Chaban Maria
slide 2
organic matter
- Some organic substances have been known to man for many decades, others are at the stage of study, and still others are just waiting in the wings. But one thing is certain: organic chemistry can never exhaust itself. Its diversity is hidden in its nature.
slide 3
- I consider it important to convey the understanding that food products, clothing, footwear, medicines, dyes, building parts, electrical, radio and television equipment, synthetic fibers, plastics and rubber, means of increasing productivity, explosives - this is an incomplete list of what gives organic chemistry to man.
slide 4
- The chemical and petrochemical industries are the most important industries, without which the functioning of the economy is impossible. Among the most important products of chemistry are acids, alkalis, salts, mineral fertilizers, solvents, oils, plastics, rubbers and rubbers, synthetic fibers and much more. Currently, the chemical industry produces several tens of thousands of products.
slide 5
Competing with nature, organic chemists have created a large number of compounds that have properties that are necessary and useful for people. These are organic dyes, in diversity and beauty far superior to natural ones; a huge arsenal of medicines that help a person overcome various diseases; synthetic detergents that ordinary soap cannot compete with, and much more. All these substances have penetrated into our lives so much that a person can no longer imagine his existence without them.
slide 6
Medicine and chemistry
- Chemistry plays an important role in the development of the pharmaceutical industry: the bulk of all drugs are obtained synthetically. Thanks to chemistry, many revolutions in medicine have been made. Without chemistry, we would not have painkillers, sleeping pills, antibiotics and vitamins. This certainly does chemistry credit. Chemistry also helped to cope with unsanitary conditions, because back in the 18th century. doctor I. Zimmelweis ordered the medical staff of the hospital to wash their hands in bleach solution. Mortality of patients has sharply decreased.
Slide 7
Industry and chemistry
- The development of many industries is associated with chemistry: metallurgy, engineering, transport, industry building materials, electronics, light industry, food industry - this is an incomplete list of economic sectors that widely use chemical products and processes. In many industries, chemical methods are used, for example, catalysis (acceleration of processes), chemical treatment metals, protection of metals from corrosion, water treatment.
Slide 8
- Organic chemistry allows a person to conquer long distances, providing his vehicles (cars, ships and planes) with fuel and lubricants.
Slide 9
Chemistry and plastics
In the automotive industry, the use of plastics for the manufacture of cabins, bodies and their large-sized parts is especially promising. the bodywork accounts for about half of the car's mass and ~40% of its cost. Plastic bodies are more reliable and durable than metal ones, and their repair is cheaper and easier. However, plastic masses have not yet received wide distribution in the production of large-sized car parts, mainly due to insufficient rigidity and relatively low weather resistance. The most widely used plastic masses for interior trim of the car.
Slide 10
- Plastics are also used to make engine, transmission, and chassis parts. The enormous importance that plastics play in electrical engineering is determined by the fact that they are the basis or an indispensable component of all insulation elements of electrical machines, apparatus and cable products. Plastic masses are also often used to protect insulation from mechanical stress and aggressive environments, and for the manufacture of structural materials.
slide 11
- The trend towards an ever wider use of plastics (especially film materials) is characteristic of all countries with developed agriculture. They are used in the construction of cultivation facilities, for soil mulching, seed coating, packaging and storage of agricultural products. products, etc. In melioration and page - x. water supply, polymer films serve as screens that prevent water loss for filtration from irrigation canals and reservoirs; pipes for various purposes are made from plastics, they are used in the construction of water facilities
slide 12
- Unfortunately, organic chemistry is not only a good friend and magician. Often, by the will of people or by chance, it turns into its opposite - destructive chemistry. This happens if a person treats it carelessly, illiterately or with malicious intent.
- Growth environmental issues- a sad retribution for the numerous mistakes and mistakes of people producing organic substances or working with them. In addition, organic chemistry is not only a source of products necessary for humans.
slide 13
- Drugs, carcinogens, chemical warfare agents, the filling of mines, grenades, bombs and shells are also organic substances. Therefore, we must not allow organic chemistry to work against us.
View all slides
The topic “Subject of Organic Chemistry. Role organic matter In human life". The teacher highlights the question of why it became necessary to separate substances into organic and inorganic. Then he tells students about the carbon cycle in nature, defines organic substances, explains what derivatives of hydrocarbons, organogens are. At the end of the lesson, the teacher will reveal the role of organic chemistry in our lives.
Topic: Introduction to organic chemistry
Lesson: The subject of organic chemistry.The role of organic substances in human life
By the beginning of the 21st century, chemists had isolated millions of substances in their pure form. At the same time, more than 18 million compounds of carbon and less than a million compounds of all other elements are known.
Carbon compounds are mainly classified as organic compounds.
Substances began to be divided into organic and inorganic with early XIX century. At that time, substances isolated from animals and plants were called organic, and inorganic - extracted from minerals. It is through the organic world that the main part of the carbon cycle in nature passes.

From compounds containing carbon, to inorganic traditionally include graphite, diamond, carbon oxides (CO and CO 2), carbonic acid(H 2 CO 3), carbonates (for example, sodium carbonate - soda Na 2 CO 3), carbides (calcium carbide CaC 2), cyanides (potassium cyanide KCN), thiocyanates (sodium thiocyanate NaSCN).
A more precise modern definition: organic compounds are hydrocarbons and their derivatives.
The simplest hydrocarbon is methane. Carbon atoms are able to connect with each other, forming chains of any length. If in such chains carbon is also bonded to hydrogen, the compounds are called hydrocarbons. Tens of thousands of hydrocarbons are known.

Models of molecules of methane CH 4, ethane C 2 H 6, pentane C 5 H 12
Hydrocarbon derivatives are hydrocarbons in which one or more hydrogen atoms are replaced by an atom or group of atoms of other elements. For example, one of the hydrogen atoms in methane can be replaced by chlorine, or by an OH group, or by an NH 2 group.

Methane CH 4, chloromethane CH 3 Cl, methyl alcohol CH 3 OH, methylamine CH 3 NH 2
The composition of organic compounds, in addition to carbon and hydrogen atoms, may include atoms of oxygen, nitrogen, sulfur, phosphorus, less often halogens.
To appreciate the importance of the organic compounds that surround us, imagine that they suddenly disappeared. There are no wooden objects, books and notebooks, no bags for books and ballpoint pens. The plastic cases of computers, televisions and other household appliances have disappeared, there are no telephones and calculators. Transport stopped without gasoline and diesel fuel, most of the medicines are missing and there is simply nothing to eat. There are no detergents, clothes, and even us ...

There are so many organic substances due to the peculiarities of formation chemical bonds carbon atoms. These small atoms are able to form strong covalent bonds with each other and with organogenic non-metals.
In the ethane molecule C 2 H 6, 2 carbon atoms are bonded to each other, in the pentane molecule C 5 H 12 - 5 atoms, and in the well-known polyethylene molecule hundreds of thousands of carbon atoms.
The structure, properties and reactions of organic substances studies organic chemistry.
Chemistry. Grade 10. Profile level: studies. for general education Institutions / V.V. Eremin, N.E. Kuzmenko, V.V. Lunin. – M.: Bustard, 2008. – 463 p.
ISBN 978-5-358-01584-5
Chemistry. Grade 11. Profile level: textbook. for general education Institutions / V.V. Eremin, N.E. Kuzmenko, V.V. Lunin. – M.: Bustard, 2010. – 462 p.
Khomchenko G.P., Khomchenko I.G. Collection of problems in chemistry for those entering the universities. - 4th ed. - M.: RIA "New Wave": Publisher Umerenkov, 2012. - 278 p.
online tutorial
Samara State University.
Department of Organic, Bioorganic and Medicinal Chemistry
The importance of organic chemistry in people's lives
Chemistry teacher
MOU "Secondary School No. 41"
Saratov
Vinnik Nina Arnoldovna
2015
Introduction Everywhere we are surrounded by objects and products made from substances and materials obtained at chemical plants and factories. In addition, in everyday life, without knowing it, each person carries out chemical reactions. For example, washing with soap, washing with detergents, etc. Lighting a match, mixing sand and cement with water, burning bricks, we carry out real, and sometimes quite complex chemical reactions. Cooking is also a chemical process. It should only be noted that in any living organism various chemical reactions are carried out in huge quantities. The processes of digestion of food, respiration of animals and humans are based on chemical reactions. Organic chemistry - chemistry of carbon compounds|
The most important characteristics of organic compounds |
Notes |
(about 27 million) |
Inorganic several hundred thousand |
H and C atoms |
All organic compounds are combustible, gas and water are formed during combustion. |
|
Most have a molecular crystal lattice |
(in solution as molecules) |
Reactions proceed slowly and more often with the participation of a catalyst |
|
Proteins, fats, carbohydrates, nucleic acids |
- Hydrogen peroxide (H2O2) is an excellent antiseptic.
- Ammonia (an aqueous solution of ammonia NH3) excites the respiratory center.
- Aspirin, or acetylsalicylic acid, is one of the drugs that is widely used as an antipyretic, anti-inflammatory, analgesic and antirheumatic agent.
- Medicines for treatment of cardio-vascular system- this is validol, corvalol, nitro glycerin.
- Means for the treatment of the digestive system.
- Antibiotics.
- Vitamins - a means of strengthening body, increase the general tone, resistance to diseases
- Medications- potent agents.
- The pearlescent effect in cosmetics is created by bismuthyl salts BiOCl and BiO(NO3) or titanized mica - pearlescent powder containing about 40% TiO2. Zinc oxide ZnO is used to create special cosmetics (make-ups). In medicine, it is used in powders and for the manufacture of ointments.
- Diluted dyes are used as hair dye. aqueous solutions highly soluble salts of lead, silver, copper, bismuth.
- Hair lightening is done with a 3% hydrogen peroxide solution.
- Coloring shampoos contain P- phenylenediamine, resorcinol and other similar compounds.
- Fats make up an essential part of our food. They are found in meat, fish, dairy products, grains. Components of natural fat, important of which are phosphatides, sterols, vitamins, pigments and odor carriers.
- Phosphatides are, in fact, also esters, but they contain residues phosphoric acid and amino alcohol.
- Sterols are natural polycyclic compounds of a very complex configuration. The representative is cholesterol.
- Vitamins. They are rich in fish liver and sea animal, vegetable fats, and butter.
- Pigments are substances that give color to fats.
- The odor carriers are very diverse and complex in structure, there are more than 20 of them in the composition of butter.
- Glucose is a monosaccharide (C6H12O6). Glucose is easily absorbed by the body. Glucose is found in fruits and berries.
- Fructose (C6H12O6) is also a monosaccharide, an isomer of glucose.
- Sucrose is a disaccharide (C12H22O11). In real life, it's just sugar.
- Lactose is a disaccharide (C12H22O11) Mainly found in animal milk.
- Starch-polysaccharide ((C6H10O5) n) is the main carbohydrate in food. Found in potatoes and grains.
- Glycogen ("animal starch")
- Cellulose ((C6H10O5)n) is a plant polysaccharide. Enters the body with plant foods.
- E100-E182- dyes
- E200- E299 - preservatives
- E300-E399 - substances that slow down the processes of fermentation and oxidation in food
- E400-E409 - stabilizers (provide long-term preservation of consistency)
- E500- E599 - emulsifiers
- E600-E699 - flavorings (enhance or add flavor to food products)
- E900-E999 - antiflaming agents that prevent flour, granulated sugar, salt, soda, citric acid, dough baking powder from caking, as well as substances that prevent the formation of foam in drinks.
- Chemistry, possessing enormous possibilities, creates unprecedented materials, multiplies the fertility of the soil, facilitates the work of a person, saves his time, clothes, preserves his health, creates coziness and comfort for him, changes the appearance of people. But the same chemistry can become dangerous to human health, even deadly.
- Any chemical pollution is the appearance of a chemical in a place not intended for it. Pollution arising from human activity is the main factor in its harmful impact on the natural environment.
- Chemical pollutants can cause acute poisoning, chronic diseases, and also have carcinogenic and mutagenic effects.
- Take a look around and you will see that the life of a modern person is impossible without chemistry. Even in ancient times, long before the birth of Christ, man observed chemical phenomena in nature and tried to use them to improve the conditions of his existence. Souring of milk, fermentation of the sweet juice of fruits, the action of poisonous plants attracted the attention of a person. We use chemicals in food production. We move in cars, their metal, rubber and plastic are made using chemical processes. We use perfumes, eau de toilette, soaps and deodorants, the production of which is unthinkable without chemicals. There is even an opinion that the most exalted feeling of a person, love, is a set of certain chemical reactions in the body.
- http://www.krugosvet.ru/enc/nauka_i_tehnika/himiya/HIMIYA_ORGANICHESKAYA.html
- http://www.chemistry2011.ru/chemistry_-_our_life/
- Lit .: Chalmers L., Chemicals in everyday life and industry, trans. from English, L., 1969;
- Zhdanov Yu. A. Carbon and life. - Rostov-on-Don, 1968, p. eighteen.
The development of the chemical industry brings human life to a completely new qualitative level. However, most people consider chemistry to be very complex and impractical science engaged in abstract things that are completely unnecessary in life. Let's try to dispel this myth.
In contact with
Why does humanity need chemistry
The role of chemistry in modern world very large. In fact, chemical processes surround us all the time, this applies not only to industrial production or domestic moments.
Chemical reactions in our own bodies take place every second, breaking down organic matter into simple compounds like carbon dioxide and , as a result of which we get energy to perform elementary actions.
In parallel, we create new substances necessary for the life and work of all organs. Processes stop only after the death of a person and its complete destruction.
The food source for many organisms, including humans, are plants that have the ability to produce organic substances from water and carbon dioxide.
This process includes chain of complex chemical transformations, the result of which is the formation of biopolymers: fiber, starch, cellulose.
Attention! How fundamental science, chemistry is engaged in the formation of ideas about the world, about the relationships in it, the unity of discrete and continuous.
Chemistry at home
Chemistry in human life is present daily, we are faced with the implementation of a whole chain of chemical transformations with:
- using soap;
- making tea with lemon
- extinguishing soda;
- lighting a match or gas burner;
- cooking sauerkraut;
- using powders and other detergents.
All these are chemical reactions, during which others are formed from some substances, and a person receives some benefit from this process. Modern powders contain enzymes that decompose at high temperatures, so washing in hot water is impractical. The effect of eating away spots will be minimal.
The action of soap in hard water is also significantly reduced, but flakes appear on the surface. You can soften the water by boiling, but sometimes this is only possible with the help of chemical substances, which are just added to the means for the washing machine, which reduce the process of scale formation.
Chemistry and the human body
 The role of chemistry in human life begins from breathing and digestion.
The role of chemistry in human life begins from breathing and digestion.
All processes occurring in our body are carried out in a dissolved form, and water acts as a universal solvent. Its magical properties once allowed origin of life on earth and are now very important.
The basis of the chemical structure of a person is the food that he consumes. The better and more complete it is, the better the well-coordinated mechanism of life functions.
With a lack of any substance in the diet, processes are slowed down and the functioning of the organism is disturbed. Most often, such important substances we count vitamins. But these are the most noticeable substances, the lack of which manifests itself quickly. The lack of other components may not be as visible.
For example, vegetarianism has negative aspects associated with the lack of food intake of some complete proteins and the amino acids contained in them. In such a situation, the body cannot synthesize some of its own proteins, which leads to various violations.
Even table salt must be included in the diet, since its ions help to carry out osmotic pressure, are part of the gastric juice, help work.
With various deviations in the activity of organs and systems, a person first of all turns to a pharmacy, which acts as the main promoter of human achievements in the field of chemistry.
More than 90 percent of medicines displayed on the shelves of pharmacies are artificially synthesized, even if they are present in nature, today it is easier to create them in a factory from individual components than to grow them in natural conditions. And although many of them have a side effect, the positive value of eliminating the disease is much higher.
Attention! Cosmetology is almost entirely built on the achievements of chemists. It allows you to prolong the youth and beauty of a person, while simultaneously bringing substantial income to cosmetic companies.
Chemistry at the service of industry
 Initially, the science of chemistry was driven by people who were curious, as well as greedy.
Initially, the science of chemistry was driven by people who were curious, as well as greedy.
The former were interested in knowing what everything consists of and how it turns into something new, the latter wanted to learn how to create something valuable that would allow them to acquire material wealth.
One of the most valuable substances is gold, followed by others.
Exactly mining and processing of ore for the production of metals - the first directions in the development of chemistry, they are very important today. Because they allow get new alloys, use more effective ways metal cleaning and so on.
The production of ceramics and porcelain is also very ancient, it is gradually being improved, although it is difficult to surpass some of the old masters.
Oil refining today shows a huge h the meaning of chemistry, because in addition to gasoline and other types of fuel, several hundred different substances are created from this natural raw material:
- rubbers and rubbers;
- synthetic fabrics such as nylon, lycra, polyester;
- car parts;
- plastics;
- detergents and household chemicals;
- plumbing;
- stationery;
- furniture;
- toys;
- and even food.
The paint and varnish industry is completely based on the achievements of chemistry, all its diversity is created by scientists, synthesizing new substances. Even construction today is using with might and main new materials that have properties that are uncharacteristic of natural substances. Their quality is gradually improving, proving that chemistry is necessary in human life.
Two sides of the coin
The role of chemistry in the modern world is enormous, we can no longer live without it, it gives us a lot of useful substances and phenomena, but at the same time it causes certain harm.
Harmful effects of chemicals
 As a negative factor, chemistry in a person's life appears constantly. Most often we celebrate environmental impact and public health.
As a negative factor, chemistry in a person's life appears constantly. Most often we celebrate environmental impact and public health.
The abundance of materials alien to our planet leads to the fact that they contaminate soil and water without being subjected to natural decay processes.
At the same time, during decomposition or combustion, they release a large amount of toxic substances, additionally poisonous environment.
And yet, this question is quite resolvable with the help of the same chemistry.
A significant part of the substances can recycle, again turning into the desired goods. The problem, rather, is connected not with the shortcomings of chemistry as a science, but with the laziness of a person, and his unwillingness to spend extra effort for the processing of waste products.
The same problem is associated with industrial waste, which today is rarely processed efficiently, poisoning the environment and human health.
The second point, saying that chemistry and the human body are incompatible, is artificial food, which many manufacturers are trying to stuff us with. But here the question is not so much the achievements of chemistry as the greed of people.
Chemical advances make human life easier, and perhaps the role of chemistry in solving the food problem will be invaluable, especially when combined with the achievements of genetics. The inability to use these achievements and the desire to earn - that's the main enemies of human health rather than the chemical industry.
The use of a large number of preservatives in food has become a problem in some countries, where the inhabitants are so saturated with these substances that, after death, the processes of decomposition in them are greatly inhibited, as a result the dead just don't rot and lie in the ground for many years.
Household chemicals often become a source allergic reactions and poisoning organism. Mineral fertilizers and means for treating plants from pests are also dangerous for humans, and they are also harmful to nature. render negative impact gradually destroying it.
The benefits of chemistry
 In psychology, there is such a concept - which consists in removing internal stress through redistribution, to achieve a result in some available area.
In psychology, there is such a concept - which consists in removing internal stress through redistribution, to achieve a result in some available area.
In chemistry, this term is used as a designation for the process of obtaining a gaseous substance from a solid without a liquid stage. However, in this industry, you can apply the approach of psychology.
Redirecting energy towards achievements in various industries related to chemistry brings a lot benefit to society.
Speaking about why chemistry is needed in human life or industrial production, we recall many of its achievements that have made our life comfortable and longer:
- medicines;
- modern materials with unique properties;
- fertilizers;
- energy sources;
- food sources and more.
Chemistry in human life
If chemistry didn't exist. Why Study Chemistry
Conclusion
The role of chemistry in the modern world is undeniable, it occupied an important place in the system of human knowledge accumulated over millennia. Its active development in the 20th century is somewhat frightening and makes people think about the ultimate goal of applying their knowledge. But without knowledge, humanity is only a separate group of individuals with not the best characteristics.
Chemistry finds application in various branches of human activity - medicine, agriculture, production of ceramic products, varnishes, paints, automotive, textile, metallurgical and other industries. In everyday life, chemistry is reflected primarily in various subjects. household chemicals(detergents and disinfectants, care products for furniture, glass and mirror surfaces, etc.), medicines, cosmetics, various plastic products, paints, adhesives, insecticides, fertilizers, etc. This list can be continued almost endlessly, we will consider only some of its points.
Household chemicals
Of the household chemicals, the first place in terms of production and use is occupied by detergents, among which various soaps, washing powders and liquid detergents (shampoo and gels) are the most popular.
Soaps are mixtures of salts (potassium or sodium) of unsaturated fatty acids (stearic, palmitic, etc.), with sodium salts forming solid soaps, and potassium salts forming liquid ones.
Soaps are obtained by the hydrolysis of fats in the presence of alkalis (saponification). Consider the production of soap using the example of saponification of tristearin (triglyceride of stearic acid):
where C 17 H 35 COONa is soap - the sodium salt of stearic acid (sodium stearate).
It is also possible to obtain soap using alkyl sulfates (salts of esters of higher alcohols and sulfuric acid) as raw materials:
R-CH 2 -OH + H 2 SO 4 \u003d R-CH 2 -O-SO 2 -OH (sulfuric acid ester) + H 2 O
R-CH 2 -O-SO 2 -OH + NaOH \u003d R-CH 2 -O-SO 2 -ONa (soap - sodium alkyl sulfate) + H 2 O
Depending on the scope of application, household, cosmetic (liquid and solid) soaps, as well as handmade soaps are distinguished. In addition, various flavors, dyes or fragrances can be added to the soap.
Synthetic detergents (washing powders, gels, pastes, shampoos) are complex chemical composition mixtures of several components, the main component of which are surface-active substances (surfactants). Among surfactants, ionic (anionic, cationic, amphoteric) and nonionic surfactants are distinguished. For the production of synthetic detergents, inogenic anionic surfactants are usually used, which are alkyl sulfates, amino sulfates, sulfosuccinates, and other compounds that dissociate into ions in an aqueous solution.
Powder detergents usually contain various additives to remove grease. Most often it is soda ash or drinking soda, sodium phosphates.
Chemical bleaches are added to some powders - organic and inorganic compounds, during the decomposition of which active oxygen or chlorine is released. Sometimes, enzymes are used as bleaching additives, which, due to the rapid process of protein breakdown, remove organic contaminants well.
Polymer products
Polymers are high-molecular compounds whose macromolecules consist of "monomeric units" - molecules of inorganic or organic substances connected by chemical or coordination bonds.
Products made of polymers are widely used in the daily life of mankind - these are all kinds of household items - kitchen utensils, bathroom items, household appliances, storage containers, packaging materials, etc. Polymer fibers are used to make a variety of fabrics, knitwear, hosiery, artificial fur curtains, carpets, upholstery materials for furniture and cars. Synthetic rubber is used to produce rubber products (boots, galoshes, sneakers, rugs, shoe soles, etc.).
Among the many polymeric materials, polyethylene, polypropylene, polyvinyl chloride, teflon, polyacrylate and foam are widely used.
Among polyethylene products, the most famous in everyday life are polyethylene film, all kinds of containers (bottles, cans, boxes, canisters, etc.), pipes for sewerage, drainage, water, gas supply, armor, heat insulators, hot-melt adhesive, etc. All these products are made from polyethylene, obtained in two ways - at high (1) and low pressure (2):


DEFINITION
Polypropylene is a polymer obtained by polymerization of propylene in the presence of catalysts (for example, a mixture of TiCl 4 and AlR 3):
n CH 2 \u003d CH (CH 3) → [-CH 2 -CH (CH 3) -] n
This material has found wide application in the production of packaging materials, household items, non-woven materials, disposable syringes, in construction for vibration and noise insulation of interfloor ceilings in "floating floor" systems.
Polyvinyl chloride (PVC) is a polymer obtained by suspension or emulsion polymerization of vinyl chloride, as well as bulk polymerization:
It is used for the electrical insulation of wires and cables, the production of sheets, pipes, films for stretch ceilings, artificial leather, linoleum, profiles for the manufacture of windows and doors.
Polyvinyl chloride is used as a sealant in domestic refrigerators, instead of relatively complex mechanical seals. PVC is also used to make condoms for people with latex allergies.
Cosmetics
The main products of cosmetic chemistry are all kinds of creams, lotions, face, hair and body masks, perfumes, eau de toilette, hair dyes, mascaras, hair and nail polishes, etc. The composition of cosmetic products includes substances that are contained in the tissues for which these products are intended. So, cosmetic preparations for nail, skin and hair care include amino acids, peptides, fats, oils, carbohydrates and vitamins, i.e. substances necessary for the life of the cells that make up these tissues.
In addition to substances obtained from natural raw materials (for example, various plant extracts), synthetic raw materials are widely used in the production of cosmetics, which are obtained by chemical (often organic) synthesis. Substances obtained in this way are characterized a high degree purity.
The main types of raw materials for the production of cosmetics are natural and synthetic animal (chicken, mink, pig) and vegetable (cotton, linseed, castor oil) fats, oils and waxes, hydrocarbons, surfactants, vitamins and stabilizers.