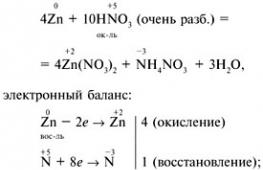Obtaining and properties of oxygen. Gaseous substances in inorganic and organic chemistry Gas can be collected by the air displacement method
Test "Nitrogen and its compounds"
Option 1 1. The strongest molecule a) H 2; b) F 2 ; c) O 2; d) N 2 . 2. Phenolphthalein color in ammonia solution: a) crimson; b) green; c) yellow; d) blue. 3. The oxidation state is +3 at the nitrogen atom in the compound: a) NH 4 NO 3; b) NaNO 3 ; c) NO 2; d) KNO 2. 4. During thermal decomposition of copper (II) nitrate, the following are formed:a) copper (II) nitrite and O 2 ;b) nitric oxide(IV) and O 2 ;c) copper(II) oxide, brown gas NO 2 and O 2 ; d) copper (II) hydroxide, N 2 and O 2. 5. Which ion is formed by the donor-acceptor mechanism? a) NH 4 + ; b) NO 3 - ; c) Cl - ; d) SO 4 2–. 6. Specify strong electrolytes: a) nitric acid; b) nitrous acid; c) an aqueous solution of ammonia; d) ammonium nitrate. 7. Hydrogen is released during the interaction: a) Zn + HNO 3 (razb.); b) Cu + HCl (solution); c) Al + NaOH + H 2 O; d) Zn + H 2 SO 4 (razb.); e) Fe + HNO 3 (conc.). 8. Write an equation for the reaction of zinc with very dilute nitric acid if one of the reaction products is ammonium nitrate. Specify the coefficient in front of the oxidizing agent. 9.Name the substances A, B, C. Option 2 1. It is impossible to collect by the method of displacement of water: a) nitrogen; b) hydrogen; c) oxygen; d) ammonia. 2. The reagent for the ammonium ion is a solution of: a) potassium sulfate; b) silver nitrate; c) sodium hydroxide; d) barium chloride. 3. When interacting with HNO 3 (conc.) gas is formed with copper shavings: a) N 2 O; b) NH 3; c) NO 2 ; d) H 2 . 4. Thermal decomposition of sodium nitrate produces: a) sodium oxide, brown gas NO 2, O 2; b) sodium nitrite and O 2; c) sodium, brown gas NO 2, O 2; d) sodium hydroxide, N 2, O 2. 5. The degree of nitrogen oxidation in ammonium sulfate: a) -3; b) -1; c) +1; d) +3. 6. With which of the following substances does concentrated HNO react? 3 under normal conditions? a) NaOH; b) AgCl; c) Al; d) Fe; e) Cu. 7. Specify the number of ions in the abbreviated ionic equation for the interaction of sodium sulfate and silver nitrate: a) 1; b) 2; in 3; d) 4. 8. Write an equation for the interaction of magnesium with dilute nitric acid if one of the reaction products is a simple substance. Specify the coefficient in the equation in front of the oxidizing agent. 9. Write reaction equations for the following transformations:
Name the substances A, B, C, D.
Answers
Option 1 1 - G; 2 - but; 3 - G; 4 - in; 5 - but; 6 - a, d; 7 - c, d; 8 – 10,


2Ag + + SO 4 2– = Ag 2 SO 4;
8 – 12, 9. A - NO, B - NO 2, C - HNO 3, D - NH 4 NO 3,
Gathering gases
The methods of collecting gases are determined by their properties: solubility and interaction with water, air, poisonousness of the gas. There are two main methods of gas collection: air displacement and water displacement. Air displacement collect gases that do not interact with air.
According to the relative density of gas in air, a conclusion is made on how to position the vessel for collecting gas (Fig. 3, a and b).
On fig. 3a shows the collection of a gas with an air density greater than unity, such as nitric oxide (IV), whose air density is 1.58. On fig. 3b shows the collection of gas with an air density of less than unity, such as hydrogen, ammonia, etc.
By displacing water, gases are collected that do not interact with water and are poorly soluble in it. This method is called collecting gas above the water , which is carried out as follows (Fig. 3, c). The cylinder or jar is filled with water and covered with a glass plate so that no air bubbles remain in the cylinder. The plate is held by hand, the cylinder is turned over and lowered into a glass bath of water. Under water, the plate is removed, a gas outlet tube is brought into the open hole of the cylinder. The gas gradually displaces water from the cylinder and fills it, after which the hole of the cylinder under water is closed with a glass plate and the cylinder filled with gas is removed. If the gas is heavier than air, then the cylinder is placed upside down on the table, and if it is lighter, then upside down on the plate. Gases above the water can be collected in test tubes, which, like the cylinder, are filled with water, closed with a finger and overturned into a glass or glass bath with water.
Toxic gases are usually collected by displacing water, since it is easy to note the moment when the gas completely fills the vessel. If there is a need to collect gas by the method of air displacement, then for this proceed as follows (Fig. 3, d).
A cork with two gas outlet tubes is inserted into the flask (jar or cylinder). Through one, which reaches almost to the bottom, gas is let in, the end of the other is lowered into a glass (jar) with a solution that absorbs gas. So, for example, to absorb sulfur oxide (IV), an alkali solution is poured into a glass, and water is poured into a glass to absorb hydrogen chloride. After filling the flask (jar) with gas, the cork with gas outlet tubes is removed from it and the vessel is quickly closed with a cork or glass plate, and the cork with gas outlet tubes is placed in a gas-absorbing solution.
Experience 1. Obtaining and collecting oxygen
Assemble the installation according to fig. 4. Place 3-4 g of potassium permanganate into a large dry test tube, close with a stopper with a gas outlet tube. Fix the test tube in the rack obliquely with the hole slightly up. Next to the tripod on which the test tube is mounted, place the crystallizer with water. Fill an empty test tube with water, close the hole with a glass plate and quickly turn it upside down into the crystallizer. Then in the water, take out the glass plate. There should be no air in the test tube. Heat potassium permanganate in a burner flame. Dip the end of the gas outlet tube into the water. Observe the appearance of gas bubbles.
A few seconds after the start of bubbles, put the end of the gas outlet tube into the hole of the test tube filled with water. Oxygen displaces water from the tube. After filling the test tube with oxygen, cover its opening with a glass plate and turn it upside down.
1. What laboratory methods for obtaining oxygen do you know? Write the corresponding reaction equations.
2. Describe your observations. Explain the location of the test tube during the experiment.
3. Write an equation for the chemical reaction of the decomposition of potassium permanganate when heated.
4. Why does a smoldering splinter flare up in a test tube with oxygen?
Experience 2. Hydrogen production the action of a metal on an acid
Assemble the apparatus, consisting of a test tube with a stopper, through which a glass tube with a retracted end passes (Fig. 5). Place a few pieces of zinc in a test tube and add a dilute solution of sulfuric acid. Firmly insert the stopper with the tube pulled back, fix the test tube vertically in the tripod clamp. Observe gas evolution.
If air is present in a test tube with hydrogen, a small explosion occurs, accompanied by a sharp sound. In this case, the gas purity test should be repeated. After making sure that pure hydrogen comes out of the device, light it at the hole of the drawn tube.
Control questions and tasks:
1. Specify the methods of obtaining and collecting hydrogen in the laboratory. Write the corresponding reaction equations.
2. Write an equation for the chemical reaction to produce hydrogen under experimental conditions.
3. Hold a dry tube over the hydrogen flame. What substance is produced by burning hydrogen? Write the equation for the hydrogen combustion reaction.
4. How to check the purity of the hydrogen obtained during the experiment?
Experience 3. Getting ammonia
Control questions and tasks:
1. What hydrogen compounds of nitrogen do you know? Write their formulas and names.
2. Describe what is happening. Explain the location of the test tube during the experiment.
3. Write an equation for the reaction between ammonium chloride and calcium hydroxide.
Experience 4. Obtaining nitric oxide (IV)
Assemble the device according to fig. 7. Put some copper shavings into the flask, pour 5-10 ml of concentrated nitric acid into the funnel. Pour acid into the flask in small portions. Collect the escaping gas in a test tube.
Control questions and tasks:
1. Describe what is happening. What is the color of the escaping gas?
2. Write an equation for the reaction of the interaction of copper with concentrated nitric acid.
3. What properties does nitric acid have? What factors determine the composition of the substances to which it is reduced? Give examples of reactions between metals and nitric acid, as a result of which the products of HNO 3 reduction are NO 2 , NO, N 2 O, NH 3 .
Experience 5. Getting hydrogen chloride
Place 15-20 g of sodium chloride in a Wurtz flask; into a dropping funnel - a concentrated solution of sulfuric acid (Fig. 8). Insert the end of the gas outlet tube into a dry vessel for collecting hydrogen chloride so that the tube reaches almost to the bottom. Close the opening of the vessel with a loose ball of cotton wool.
Place a crystallizer with water next to the device. Pour the sulfuric acid solution from the dropping funnel.
Warm the flask slightly to speed up the reaction. When over
cotton wool, with which the opening of the vessel is closed, fog will appear,
Control questions and tasks:
1. Explain the observed phenomena. What is the reason for the formation of fog?
2. What is the solubility of hydrogen chloride in water?
3. Test the resulting solution with litmus paper. What is the pH value?
4. Write the equation for the chemical reaction of the interaction of solid sodium chloride with concentrated sulfuric acid.
Experience 6. Obtaining and collecting carbon monoxide (IV)
The installation consists of a Kipp apparatus 1 , charged with pieces of marble and hydrochloric acid, two Tishchenko flasks connected in series 2 And 3 (bottle 2 filled with water to purify passing carbon monoxide (IV) from hydrogen chloride and mechanical impurities, bottle 3 - sulfuric acid for gas drying) and flasks 4 with a capacity of 250 ml for collecting carbon monoxide (IV) (Fig. 9).
Rice. 9. Device for obtaining carbon monoxide (IV)
Control questions and tasks:
1. Lower the lit torch into a flask with carbon monoxide (IV) and explain why the flame goes out.
2. Write an equation for the formation of carbon monoxide (IV).
3. Is it possible to use a concentrated solution of sulfuric acid to obtain carbon monoxide (IV)?
4. Pass the gas released from the Kipp apparatus into a test tube with water, tinted with a neutral solution of litmus. What is observed? Write the equations for the reaction that occurs when a gas is dissolved in water.
Test questions:
1. List the main characteristics of the gaseous state of matter.
2. Propose a classification of gases according to 4-5 essential features.
3. How is Avogadro's law read? What is its mathematical expression?
4. Explain the physical meaning of the average molar mass of the mixture.
5. Calculate the average molar mass of conditional air, in which the mass fraction of oxygen is 23%, and nitrogen is 77%.
6. Which of the following gases is lighter than air: carbon monoxide (II), carbon monoxide (IV), fluorine, neon, acetylene C 2 H 2, phosphine PH 3?
7. Determine the hydrogen density of a gas mixture consisting of argon with a volume of 56 liters and nitrogen with a volume of 28 liters. The volumes of gases are given to n.o.s.
8. An open vessel is heated at a constant pressure from 17 ° C to 307 ° C. What part of the air (by weight) in the vessel is displaced?
9. Determine the mass of 3 liters of nitrogen at 15 ° C and a pressure of 90 kPa.
10. The mass of 982.2 ml of gas at 100 ° C and a pressure of 986 Pa is 10 g. Determine the molar mass of the gas.
PRACTICAL WORK (1 h) Grade 8
The work is carried out by the students independently under the supervision of the teacher.
I offer the result of my many years of work on the preparation and conduct of practical work in a comprehensive school in chemistry lessons in grades 8–9:
- Obtaining and properties of oxygen,
- "Preparation of salt solutions with a certain mass fraction of the dissolved substance",
- "Generalization of information about the most important classes of inorganic compounds",
- "Electrolytic dissociation",
- "Oxygen subgroup" (see the next issue of the newspaper "Chemistry").
All of them are tested by me in the classroom. They can be used in the study of the school course of chemistry both according to the new program of O.S. Gabrielyan, and according to the program of G.E. Rudzitis, F.G. Feldman.
A student experiment is a type of independent work. The experiment not only enriches students with new concepts, skills, skills, but also is a way to verify the truth of the knowledge they have acquired, contributes to a deeper understanding of the material, the assimilation of knowledge. It allows you to more fully implement the principle of variability in the perception of the surrounding world, since the main essence of this principle is the connection with life, with the future practical activities of students.
Goals. Be able to receive oxygen in the laboratory and collect it by two methods: air displacement and water displacement; confirm experimentally the properties of oxygen; know the safety rules.
Equipment. A metal stand with a foot, a spirit lamp, matches, a test tube with a gas outlet tube, a test tube, a ball of cotton wool, a pipette, a beaker, a splinter, a dissecting needle (or wire), a crystallizer with water, two conical flasks with stoppers.
Reagents. KMnO 4 crystalline (5–6 g), Ca (OH) 2 lime water, charcoal,
Fe (steel wire or paper clip).
Safety rules.
Handle chemical equipment with care!
Remember! The test tube is heated, holding it in an inclined position, along its entire length with two or three movements in the flame of an alcohol lamp. When heating, point the opening of the test tube away from yourself and your neighbors.
Previously, students receive homework related to the study of the content of the upcoming work according to the instructions, while simultaneously using the materials of the 8th grade textbooks by O.S. Gabrielyan (§ 14, 40) or G.E. Rudzitis, F.G. Feldman (§ 19 , twenty). In notebooks for practical work, they write down the name of the topic, the goal, list the equipment and reagents, draw up a table for the report.
DURING THE CLASSES
One experience I put higher
than a thousand opinions
born only
imagination.
M.V. Lomonosov
Obtaining oxygen
air displacement method
(10 min)
1. Potassium permanganate (KMnO 4) place in a dry test tube. Place a loose ball of cotton wool at the opening of the test tube.
2. Close the test tube with a stopper with a gas outlet tube, check for tightness (Fig. 1).
|
Rice. one.
|
(Teacher's explanations on how to check the device for leaks.) Fix the device in the foot of the tripod.
3. Lower the gas outlet tube into the glass, without touching the bottom, at a distance of 2–3 mm (Fig. 2).
4. Warm up the substance in the test tube. (Remember safety regulations.)
5. Check for the presence of gas with a smoldering splinter (charcoal). What are you watching? Why can oxygen be collected by air displacement?
6. Collect the resulting oxygen in two flasks for the following experiments. Close the flasks with stoppers.
7. Prepare a report using the table. 1, which you place on the spread of your notebook.
Obtaining oxygen
water displacement method
(10 min)
1. Fill a test tube with water. Close the tube with your thumb and turn it upside down. In this position, lower the hand with the test tube into the crystallizer with water. Bring a test tube to the end of the gas outlet tube without removing it from the water (Fig. 3).
2. When the oxygen has forced the water out of the tube, close it with your thumb and remove it from the water. Why can oxygen be collected by displacing water?
Attention! Remove the gas outlet tube from the crystallizer, continuing to heat the tube with KMnO 4 . If this is not done, then the water will be thrown into a hot test tube. Why?
Combustion of coal in oxygen
(5 minutes)
1. Fix the coal on a metal wire (dissecting needle) and bring it into the flame of an alcohol lamp.
2. Lower the red-hot coal into the flask with oxygen. What are you watching? Give an explanation (Figure 4).
3. After removing the unburned coal from the flask, pour 5-6 drops of lime water into it
Ca(OH) 2 . What are you watching? Give an explanation.
4. Issue a report on the work in the table. one.
Burning steel (iron) wire
in oxygen
(5 minutes)
1. Attach a piece of a match to one end of the steel wire. Light a match. Immerse the wire with the burning match into the flask with oxygen. What are you watching? Give an explanation (Figure 5).
2. Issue a report on the work in the table. one.
Table 1
| Operations in progress (what they were doing) |
Figures with designations of initial and received substances | Observations. Terms carrying out reactions. Reaction equations |
Explanations of observations. conclusions |
|---|---|---|---|
| Assembly of the device for obtaining oxygen. Checking the device for leaks | |||
| Obtaining oxygen from KMnO 4 when heated |
|||
| Proof of oxygen production with smoldering splinter |
|||
| Characteristics of the physical properties of O 2. Collecting O 2 by two methods: air displacement, water displacement |
|||
| Characteristic chemical properties of O 2. Interaction with simple substances burning coal, burning iron (steel wire, paper clip) |
Make a written general conclusion about the work done (5 min).
OUTPUT. One of the ways to obtain oxygen in the laboratory is the decomposition of KMnO 4 . Oxygen is a colorless and odorless gas, 1.103 times heavier than air ( M r(O 2) \u003d 32, M r(air) \u003d 29, from which follows 32/29 1.103), slightly soluble in water. It reacts with simple substances, forming oxides.
Put the workplace in order (3 min): disassemble the appliance, arrange the dishes and accessories in their places.
Submit your notebooks for review.
Homework.
A task. Determine which of the iron compounds - Fe 2 O 3 or Fe 3 O 4 - is richer in iron?
| Given: | To find: |
| Fe 2 O 3, Fe 3 O 4 . |
(Fe) in Fe 2 O 3, "(Fe) to Fe 3 O 4 |
Solution
(X) = n A r(X)/ M r, where n- the number of atoms of the element X in the formula of the substance.
M r(Fe 2 O 3) \u003d 56 2 + 16 3 \u003d 160,
(Fe) \u003d 56 2/160 \u003d 0.7,
(Fe) = 70%,
M r(Fe 3 O 4) \u003d 56 3 + 16 4 \u003d 232,
"(Fe) \u003d 56 3/232 \u003d 0.724,
"(Fe) = 72.4%.
Answer. Fe 3 O 4 is richer in iron than Fe 2 O 3 .
During practical work, the teacher monitors the correctness of the performance of techniques and operations by students and notes in the skill record card (Table 2).
table 2
Skill record card
| Operations of practical work | Surnames of students | |||||
|---|---|---|---|---|---|---|
| BUT | B | IN | G | D | E | |
| Assembly of the device for obtaining oxygen | ||||||
| Checking the device for leaks | ||||||
| Fixing the test tube in the leg of the tripod | ||||||
| Alcohol lamp handling | ||||||
| Heating a test tube with KMnO 4 | ||||||
| Checking the release of O 2 | ||||||
| Collecting O 2 in a vessel by two methods: air displacement, water displacement |
||||||
| coal burning | ||||||
| Combustion of Fe (steel wire) | ||||||
| Experimental culture | ||||||
| Making work in a notebook | ||||||
Sample report on the practical work done (Table 1)
smoldering splinter
(charcoal) lights up brightly
in O 2
physical properties of O 2. Collecting O 2 by two methods:
air displacement (a),
water displacement (b)

slightly heavier than air, so
it is collected in a vessel placed on the bottom. Oxygen is slightly soluble in water


Lime water becomes cloudy, because a water-insoluble precipitate of CaCO 3 is formed:
CO 2 + Ca (OH) 2 CaCO 3 + H 2 O. Iron burns with a bright flame in oxygen:

with simple
substances - metals and non-metals. The formation of a white precipitate confirms the presence of CO 2 in the flask
CHEMISTRY
Conclusion
Task 1.
Gaseous substances are given: H2, HCl, CO2, CO, O2, NH3.
1. Determine which of them are lighter than air and which are heavier (justify the answer).
2. Determine which of them cannot be collected by water displacement.
3. Determine what will happen to these gases if they are passed through a solution of acid, alkali (confirm the answer with the reaction equations).
Solution.
1. Lighter than air, those whose molar mass is less than 29 g / mol (molar mass of air). This H 2 , CO , NH 3 . Heavier: HCl, CO 2 , O 2 .
2. The water displacement method can collect gases that are insoluble or poorly soluble in water. This H 2 , CO 2 , CO , O 2 . It is impossible to collect gases by displacement of water: HCl, NH3.
3. Substances with basic properties react with acids:
NH 3 + HCl = NH 4 Cl
Substances with acidic properties react with alkalis:
HCl + KOH = KCl + H2O
Esep 1.
Gas tarizdі zattar berіlgen: H2, HCl, CO2, CO, O2, NH3.
1.Olardyn kaysysy auadan auyr zhane kaysysy zhenіl ekenіn anyқtaңyzdar (zhauaptaryңyzdy deleldenіzder).
2. Olardyn kaysysyn courts ygystyru adіsimen anyktauga bolmaytynyn anyktanyzdar.
3. Yeger olardy sіltinіn, қyshқyldyң erіtіndіlerі аrkyly өtkіzgende osy gazdarmen ne bolatynyn anyktaңyzdar (zhauaptaryңyzdy reaction teңdeuleri ақылідідінізтер).
Sheshui.
1. Auadan zhenil, yagni molyarlyk massasy 29 g/moldan (auanyn molyarlyk massasy) kishi bolatyn gasdar: H2, CO, NH3. Auyr: HCl, CO2, O2.
2. Courts of yғgystyru adіsimen of the court of erіmeitіn nemese of the court of az eritіn gazdardy aluga bolady. Olar This is H2, CO2, CO, O2. Courts ygystyru adіsi arkyly zhinauga bolmaityn gazdar: HCl, NH3.
3. Қyshқylmen negіzdіk қasiet korsetetіn zattar аrekettesedі:
NH3 + HCl = NH4Cl
Sіltіlermen қyshқyldyқ қasiet kөrsetetіn zattar аrekettesedі:
HCl + KOH = KCl + H2O
CO2 + 2KOH = K2CO3 + H2O or CO2 + KOH = KHCO3
Task 2.
In early spring, early in the morning, when the ambient temperature was still 0 ° C, and the pressure was 760 mm Hg. Art., three comrades, walking their dogs, saw an empty bottle on the lawn. "It's empty," one of them said. "No, it's full to the brim, and I know the formula of the stuff it's filled with," said another. "You're both wrong," said the third.
1. Which of the comrades, in your opinion, was right (justify the answer)?
2. Calculate the amount of substance and the number of particles contained in the bottle if its volume is 0.7 dm3.
3. Calculate the molar mass of the gas contained in the bottle.
Solution.
1. The third one is right, because there is air in the bottle (it is not empty - the first one is wrong), and the air is not an individual substance (the second one is also wrong). Air is a mixture of gases:
2. Since the conditions are normal, thenV M = 22.4 l/mol. Calculate the amount of substancen = V / V M \u003d 0.7 / 22.4 l / mol \u003d 0.03125 mol. Number of particlesN = N A n\u003d 6.02 1023 mol-1 0.03125 mol \u003d 1.88 1022 particles.
3. The molar mass of air can be calculated by knowing the composition of the air. Air contains approximately 78% N 2 , 21% O 2 , 0.5% Ar and 0.5% CO 2 . The average molar mass will be equal toM cf = x one · M 1 + x 2 · M 2 + x 3 · M 3 + x 4 · M 4
Esep 2.
Erte koktemde tanerten erte korshagan ortanyn temperature sy 0 °C, kysym 760 mm son. bug. bolyp turgan kezde ush adam ozderinin ytterin kydyrtuғa shykty zhane olar gazondagy bos құtyny (bottle) kөrdі. "Ol boss" - grandfather onyn bireui. “Joq, auzina deyin zattarmen toly” grandfather ekіnshіsi, sebi ol құtynyң ishіndegі zattardyn formulyasyn bіladі. "Sender ekeulerin de durys tappadyndar" - grandfather ushіnshіsi.
1. Sіzderdin oylaryңyzsha, osy үsh adamnyn kaysysy dұrys oilady (zhauaptaryңdy deleldenger)?
2. Yeger құtynyn (butylkanyң) ishіndegі zattyң kolemi 0.7 dm3 - he ten bolatyn belgili bolsa, zat molsherin zhane molecularlar sanyn tabynyzdar.
3. Kutynyn ishindegi gazdyn molyarlyk massasyn eseptenіzder.
Sheshui.
1. Ushіnshi adam durys aitty, sebebі onynіshіnde aua bar (ol bos emes, endeshe birinshi adam durys tappady), al aua zheke zat emes (sol sebeptі ekіnshi adam da durys tappady). Aua birneshe gazdardyn kospasynan turady: N 2, O 2, Ar, CO 2, H 2 O, etc.
2. Yagni zhagday kalypty, endesheV M = 22.4 l/mol. Zat molsherin esepteymizn = V / V M \u003d 0.7 / 22.4 l / mol \u003d 0.03125 mol. sana moleculeN = N A n = 6,02 1023 mol-1 0.03125 mol = 1.88 1022 bolik.
3. Auanyn kuramyn bile otyryp auanyn molyarlyk massasyn esepteuge bolady. Aua shamamen tomendegi gazdar cospasynan turady: 78% N 2, 21% O 2, 0.5% Ar and 0.5% CO 2 . Ortasha molyarlyk massas ten boladasM cf = x one · M 1 + x 2 · M 2 + x 3 · M 3 + x 4 · M 4 = 0.78 28 + 0.21 32 + 0.05 40 + 0.05 44 ≈ 29 g/mol.
Task 3.
You have calcium carbonate and hydrochloric acid at your disposal. Suggest methods for the synthesis of at least 6 new substances, including 2 simple ones. In the syntheses, only the starting materials, the products of their interaction, the necessary catalysts, and electric current can be used.
Solution.
1. CaCO 3 \u003d CaO + CO 2 (when heated)
2.
3.
4. CaO + H2O = Ca(OH)2
5. CaCl 2 \u003d Ca + Cl 2 (melt electrolysis)
6. 2 HCl \u003d H 2 + Cl 2 (solution electrolysis)
7. 2H2O = 2H2 + O2 (electrolysis)
8. Ca + H2 = CaH2
9. Ca(OH)2 + Cl2 = CaOCl2 + H2O (at 0ºC)
10. when heated)
11. Cl2 + H2O = HCl + HClO (at 0ºC)
12. 3 Cl 2 + 3 H 2 O \u003d 5 HCl + HClO 3 (when heated)
Esep3.
Sizderde calcium carbonate y zhane tuz kyshkyly bar. Wasps zattar arkyly 6-dan by whom emes zhana zattardy, onyn ishinde 2 zhai zattardy kalay aluga bolady? Synthesis tek kana bastapky zattardy, olardan alyngan onnіmderdi koldanuga bolady, catalyst zhane electr togy kazhet.
Sheshui.
1. CaCO 3 \u003d CaO + CO 2 (kyzdyrganda)
2. CaCO3 + HCl = CaCl2 + CO2 + H2O
3. CaCO3 + CO2 + H2O = Ca(HCO3)2
4. CaO + H2O = Ca(OH)2
5. CaCl 2 \u003d Ca + Cl 2 (balkyma electrolysis i)
6. 2 HCl \u003d H 2 + Cl 2 (erіtndі electrolysis i)
7. 2 H 2 O \u003d 2 H 2 + O 2 (electrolysis)
8. Ca + H 2 \u003d CaH 2
9. Ca(OH)2 + Cl2 = CaOCl2 + H2O (0ºC-de)
10. 6Ca(OH)2 + 6Cl2 = 5CaCl2 + Ca(ClO3)2 + 6H2O ( kyzdyrgan kezde)
11. Cl2 + H2O = HCl + HClO (0ºC -de)
12. 3Cl2 + 3H2O = 5HCl + HClO3 (kyzdyrgan kezde)
Task 4.
A gas mixture containing two hydrogen halides has a hydrogen density of 38. The volume of this mixture at n. y. was absorbed by an equal volume of water. 11.2 ml of 0.4 mol/l sodium hydroxide solution was used to neutralize 100 ml of the resulting solution.
1. Determine which hydrogen halides could be contained in this mixture.
2. Calculate the composition of the gas mixture in volume percent.
3. Suggest a method for determining the qualitative composition of a gas mixture.
Solution.
1. Mass of 1 mol of a gas mixture at n. y. is 38 2 \u003d 76 g. Thus, in the gas mixture cannot be present simultaneously HBr and HI ( M(HBr) \u003d 81 g / mol, M(HI ) = 128 g/mol). Also, they cannot be present at the same time. HF and HCl ( M(HF) = 20 g/mol, M(HCl ) = 36.5 g/mol). The mixture must contain a hydrogen halide withMless than 76 g/mol and hydrogen halide withMmore than 76 g/mol. Possible mixture compositions: 1) HF and HBr; 2) HF and HI; 3) HCl and HBr; 4) HCl and HI.
The concentration of hydrogen halides in the solution is (11.2 0.4): 100 = 0.0448 mol/l. This value corresponds quite well to the calculated value of 1:22.4 = 0.0446 mol/l for the process of dissolving 1 liter of gas (n.a.) in 1 liter of water (provided that the hydrogen halide molecules are monomeric). Thus, the gas mixture does not contain hydrogen fluoride, which is also in the gas phase in the form ( HF ) n , where n = 2-6.
Then only two variants of mixtures correspond to the conditions of the problem: HCl + HBr or HCl + HI.
2. For a mixture of HCl + HBr: let x mole - quantity HCl in 22.4 liters of the mixture (n.a.). Then the amount HBr is (1-x ) mol. The mass of 22.4 liters of the mixture is:
36.5x + 81(1-x) = 76; x = 0.112; 1-x=0.888.
The composition of the mixture: HCl - 11.2%, HBr - 88.8%.
Similarly for a mixture HCl+HI:
36.5x + 128(1-x) = 76; x = 0.562.
Composition of the mixture: HCl - 56.2%, HI - 43.8%
3. Since both mixtures must contain hydrogen chloride, it remains to qualitatively determine hydrogen bromide or hydrogen iodide. This definition is more convenient to make in the form of simple substances - bromine or iodine. To convert hydrogen halides into simple substances, an aqueous solution can be oxidized with chlorine:
2HBr + Cl2 = 2HCl + Br2
2HI + Cl2 = 2HCl + I2
The resulting halogen solutions can be distinguished by the color of the solution in a non-polar solvent (during extraction) or by the more sensitive starch color reaction.
Also, the original hydrogen halides can be distinguished by the different color of the silver halides:
HBr + AgNO 3 = AgBr ↓ + HNO 3 (light yellow precipitate)
HI + AgNO 3 = AgI ↓ + HNO 3 (yellow precipitate)
Esep 4.
Eki halogensutekten turatyn gas kospasynyn sutek boyinsha tygyzdygy 38. Wasps kospanyn қ.zh. Alyngan 100 ml eritindin beitaraptaganda 11.2 ml 0.4 mol/l sodium hydroxydinin eritindisi jumsalda.
1. Osy kospada kandai halogensutek baryn anyktanyzdar.
2. Gas kospasynyn құramyn kolemdіk percentpen anyқtaңyzdar.
3. Gaz kospasynyn sapasyn anyktaytyn zhagdaydy usynynyzdar.
Sheshui.
1. 1 mol gas kospasynyn massasy қ.zh. kuraydy: 38 2 \u003d 76 g. M(HBr) = 81 g/mol, M(HI) = 128 g/mol) bola almaida. Sonymen katar bіr mezgіlde HF jane HCl ( M(HF) = 20 g/mol, M(HCl) = 36.5 g/mol) bola almaida. Kosapada M massasy 76g/moldan az halogensutek bolusy kerek. Mүmkin bolatyn gas cospalary: 1) HF and HBr; 2) HF instead of HI; 3) HCl instead of HBr; 4) HCl instead of HI.
Eritindidegi halosutecterdin concentrations (11.2 0.4): 100 = 0.0448 mol/l. Bulman 1 liter of suғa (halogensutec molecules monomers bulgan zhagdaida) 1 liter of gas (қ.zh.) erіtu process үshіn tөmendegi esepteu nәtizhesіne zhақyn: 1:22.4 = 0.0446 mol/l. Endeshe, gas cospasynda fluorosutek bolmaidy, sebiol gas fazasynda (HF)n turinde bolada, mundagy n = 2-6.
Endeshe eseptin sharty tek ekі nuskaga seykes keledi: HCl + HBr nemese HCl + HI.
2. HCl + HBr kospasy ushіn: 22.4 l kospadagy (қ.zh.) HCl small - x. Onda HBr younger (1-x) bolada mole. 22.4 l kospanyn massasy:
36.5x + 81(1-x) = 76; x = 0.112; 1-x=0.888.
Kospa kuramy: HCl - 11.2%, HBr - 88.8%.
Kospa Ushin HCl+HI:
36.5x + 128(1-x) = 76; x = 0.562.
Kospa kuramy: HCl - 56.2%, HI - 43.8%
3. Endeshe bromsutek zhane iodsutek ekі kospa da boluy seems. Bul anyktama zhai zat turinde - bromine nemese iod anyktauga yngayly. Halogensutekti zhai zatka aynaldyru ushіn onyn erіtіndіsіn chlormen totyқtyru kazhet:
2HBr + Cl2 = 2HCl + Br2
2HI + Cl2 = 2HCl + I2
Halogenderdin alyngan erіtіndіlerіn non-polar erіtkіshtegі erіtіndinіnіn tүsі boyinsha (extarction of kezіndegi) nemese starchdyn аserі аrkyly anyқtauға bolady.
Sondai-aқ halosutekterdi kүmіs halogenidіndegi әrtүrlі tusterі arkyly anқtauғa boladas:
HBr + AgNO3 = AgBr↓ + HNO3 (ashyk-sary tunba)
HI + AgNO3 = AgI↓ + HNO3 (sary tunba)
Problem 5 (Thermochemical calculations, impurities).
When burning 1.5 g of a zinc sample, 5.9 kJ of heat was released. Determine if the zinc sample contained non-combustible impurities if it is known that 348 kJ of heat is released when burning 1 mole of zinc.
Esep5 ( Kospalar, tyermochemiyalyk esepteuler). 1.5 g myrysh үlgisіn zhakanda 5.9 kJ zhylu bolіndі. 1 mol of myryshty zhakanda 348 kJ zhylu bөlіnetinіn bіle otyryp myrysh үlgisіnde zhanbaityn қospalar barma, zhқpa anyқtaңyzdar.
Solution:
Sheshui:
CHEMISTRY
Output
Exercise 1.
Decipher the chain of transformation and carry out chemical reactions:
 |
position:absolute; z-index:2;margin-left:218px;margin-top:91px;width:16px;height:55px">
 |
Additionally known:
Substance A– corundum
SubstanceB- the most common metal (Me) in the earth's crust
Substance C- a compound containing 15.79% Me, 28.07% S, 56.14% O
Substance E- a white gelatinous substance, poorly soluble in water. The product of the interaction of substance C with alkali
SubstanceD- the sodium salt of the most common metal, the molecule of which contains 40 electrons.
Solution:
A - Al 2 O 3
B-Al
C - Al2(SO4)3
D - NaAlO2
E – Al(OH)3
For each specific formula of the substance - 1 point
For each correct written chemical reaction equation (with implementation conditions) - 2 points
TOTAL: 5 1+8 2 = 21 points
1 tapsirma.
Ainalular tizbegin ashyp, chemistry reaction of tendeulerin zhazynyzdar:
 |
position:absolute; z-index:15;margin-left:218px;margin-top:91px;width:16px;height:55px">
 |
Kosymsha Belgili Bulgarians:
BUTzaty– corundum
Bzaty– zher sharynda en köp taralgan metal (Me)
FROM zaty - 15.79% Me, 28.07% S, 56.14% O turatyn kosylys
E zaty - ak koimalzhyn zat, courts of ours eridi. Zattyn siltimen әrekettesuinіnіn өnіmi С
D– en köp taralgan metaldyn sodium aces, molecules 40 electronnan turada.
Sheshui:
A - Al2O3
B-Al
C - Al2(SO4)3
D - NaAlO2
E – Al(OH)3
Әrbіr zattyn formulasyn anyktaғanga - 1 ұpaydan
Durys zhazylgan әrbіr chemiyalyk reaction tendeuine (sharty korsetilgen) – 2 ұpaydan
BARLYGY: 5 1 + 8 2 \u003d 21 upay
Task 2.Six numbered beakers (beakers) contain solids (in the form of powders): sodium bicarbonate, sodium chloride, zinc sulfate, potassium phosphate, calcium carbonate, iron sulfate ( II ). Using the reagents and equipment on the table, determine the contents of each vial (beaker). Give the chemical formula of each substance and write the equations of the chemical reactions carried out.
Reagents: 2 M HCl, 2 M NaOH, H 2 O distilled, 2M solution AgNO3
Equipment:rack with test tubes (7-10 pieces), spatula, pipettes.
Solution:
Stages of work | Observations | Reaction equations, conclusions |
|
Dissolve samples of substances in water | One substance did not dissolve | This is CaCO3 |
|
Add dissolved and undissolved matter to samples HCl | Gas is released in two test tubes. | NaHCO3 + HCl = CaCO3 + HCl = |
|
Add sodium hydroxide solution to sample substances (not excess) | In two test tubes precipitates are green (marsh) and white amorphous. | These are FeSO4 and Zn(NO3)2 FeSO4 + NaOH = Zn(NO3)2 + NaOH= |
|
Add silver nitrate drop by drop to the samples | In two test tubes, white curdled and yellow precipitates fall out. | These are NaCl and K3PO4 NaCl + AgNO3 = K3PO4 + AgNO3= |
For the definition of each substance, 1 point.
For the reaction equation - 2 points
Total: 6 1+6 2 = 18 points
Note: If all the coefficients are not placed in the reaction equation, but the essence of the chemical reaction is reflected - 1 point
2 tapsirms.Alty nomerlengen byukste (chemical glass) katty zat bar (ұntak turinde): sodium hydrocarbonates, sodium chloride, myryshg sulfates, potassium phosphates, calcium carbonates, temir (II) sulfates. Stoldagy reaktivterdi zhane kuraldardy paydalana otyryp, arbir byukstegi zatty anyқtaңyzdar. Әrbіr zattyn chemiyalyk formulasyn zhane himiyalyk reaction tendeulerin zhazynyzdar.
Reagent:2M HCl, 2M NaOH, distildengen H2O, 2M AgNO3 eritindis
Құral-zhabdyқtar: probirkalary bar tripod (7-10 dan), spatula (ұstagysh), pipette alar.
Sheshui:
Zhymys etaptary | Kubylys | Tendeuleri reaction |
|
Zattyn son of the masons of the court of eritu | Bir zat ta erigen zhok | Bull CaCO3 |
|
Yerigen zhane erimegen zattyn son of a masyn NSI kosu | Eki test tube gas bөlinedі | NaHCO3 + HCl = CaCO3 + HCl = |
|
Zattyn of the son of the mother's son sodium hydroxydin kosu (az molsherde) | Ekі prrobirkada zhasyl tusti (saz balshyқ tәrіzdі) zhane ak tusti amorty tunba payda bolada | Bull FeSO4 and Zn(NO3)2 FeSO4 + NaOH = Zn(NO3)2 + NaOH= |
|
Synamaga tamshylatyp kүmis nitratyn қosamyz | Ekі test tube ақ іrіmshіk tәrіzdі zhane sary tұnba tүsedі. | Bul NaCl zhane K3PO4 NaCl + AgNO3 = K3PO4 + AgNO3= |
Arbir zatty anyktaganga 1 ұpaydan.
Arbir tendeuine reaction - 2 ұpaydan.
Barlygy: 6 1+6 2 = 18Upay
Eskertu: Yeger tendeuinde barlyk reaction coefficient koyylmagan bolsa, biraq chemiyialik reaction mani anyқtalgan bolsa – 1 ұpay beruge bolada
PRACTICAL WORK (1 h) Grade 8
The work is carried out by the students independently under the supervision of the teacher.
I offer the result of my many years of work on the preparation and conduct of practical work in a comprehensive school in chemistry lessons in grades 8–9:
- Obtaining and properties of oxygen,
- "Preparation of salt solutions with a certain mass fraction of the dissolved substance",
- "Generalization of information about the most important classes of inorganic compounds",
- "Electrolytic dissociation",
- "Oxygen subgroup" (see the next issue of the newspaper "Chemistry").
All of them are tested by me in the classroom. They can be used in the study of the school course of chemistry both according to the new program of O.S. Gabrielyan, and according to the program of G.E. Rudzitis, F.G. Feldman.
A student experiment is a type of independent work. The experiment not only enriches students with new concepts, skills, skills, but also is a way to verify the truth of the knowledge they have acquired, contributes to a deeper understanding of the material, the assimilation of knowledge. It allows you to more fully implement the principle of variability in the perception of the surrounding world, since the main essence of this principle is the connection with life, with the future practical activities of students.
Goals. Be able to receive oxygen in the laboratory and collect it by two methods: air displacement and water displacement; confirm experimentally the properties of oxygen; know the safety rules.
Equipment. A metal stand with a foot, a spirit lamp, matches, a test tube with a gas outlet tube, a test tube, a ball of cotton wool, a pipette, a beaker, a splinter, a dissecting needle (or wire), a crystallizer with water, two conical flasks with stoppers.
Reagents. KMnO 4 crystalline (5–6 g), Ca (OH) 2 lime water, charcoal,
Fe (steel wire or paper clip).
Safety rules.
Handle chemical equipment with care!
Remember! The test tube is heated, holding it in an inclined position, along its entire length with two or three movements in the flame of an alcohol lamp. When heating, point the opening of the test tube away from yourself and your neighbors.
Previously, students receive homework related to the study of the content of the upcoming work according to the instructions, while simultaneously using the materials of the 8th grade textbooks by O.S. Gabrielyan (§ 14, 40) or G.E. Rudzitis, F.G. Feldman (§ 19 , twenty). In notebooks for practical work, they write down the name of the topic, the goal, list the equipment and reagents, draw up a table for the report.
DURING THE CLASSES
One experience I put higher
than a thousand opinions
born only
imagination.
M.V. Lomonosov
Obtaining oxygen
air displacement method
(10 min)
1. Potassium permanganate (KMnO 4) place in a dry test tube. Place a loose ball of cotton wool at the opening of the test tube.
2. Close the test tube with a stopper with a gas outlet tube, check for tightness (Fig. 1).
|
Rice. one.
|
(Teacher's explanations on how to check the device for leaks.) Fix the device in the foot of the tripod.
3. Lower the gas outlet tube into the glass, without touching the bottom, at a distance of 2–3 mm (Fig. 2).
4. Warm up the substance in the test tube. (Remember safety regulations.)
5. Check for the presence of gas with a smoldering splinter (charcoal). What are you watching? Why can oxygen be collected by air displacement?
6. Collect the resulting oxygen in two flasks for the following experiments. Close the flasks with stoppers.
7. Prepare a report using the table. 1, which you place on the spread of your notebook.
Obtaining oxygen
water displacement method
(10 min)
1. Fill a test tube with water. Close the tube with your thumb and turn it upside down. In this position, lower the hand with the test tube into the crystallizer with water. Bring a test tube to the end of the gas outlet tube without removing it from the water (Fig. 3).
2. When the oxygen has forced the water out of the tube, close it with your thumb and remove it from the water. Why can oxygen be collected by displacing water?
Attention! Remove the gas outlet tube from the crystallizer, continuing to heat the tube with KMnO 4 . If this is not done, then the water will be thrown into a hot test tube. Why?
Combustion of coal in oxygen
(5 minutes)
1. Fix the coal on a metal wire (dissecting needle) and bring it into the flame of an alcohol lamp.
2. Lower the red-hot coal into the flask with oxygen. What are you watching? Give an explanation (Figure 4).
3. After removing the unburned coal from the flask, pour 5-6 drops of lime water into it
Ca(OH) 2 . What are you watching? Give an explanation.
4. Issue a report on the work in the table. one.
Burning steel (iron) wire
in oxygen
(5 minutes)
1. Attach a piece of a match to one end of the steel wire. Light a match. Immerse the wire with the burning match into the flask with oxygen. What are you watching? Give an explanation (Figure 5).
2. Issue a report on the work in the table. one.
Table 1
| Operations in progress (what they were doing) |
Figures with designations of initial and received substances | Observations. Terms carrying out reactions. Reaction equations |
Explanations of observations. conclusions |
|---|---|---|---|
| Assembly of the device for obtaining oxygen. Checking the device for leaks | |||
| Obtaining oxygen from KMnO 4 when heated |
|||
| Proof of oxygen production with smoldering splinter |
|||
| Characteristics of the physical properties of O 2. Collecting O 2 by two methods: air displacement, water displacement |
|||
| Characteristic chemical properties of O 2. Interaction with simple substances burning coal, burning iron (steel wire, paper clip) |
Make a written general conclusion about the work done (5 min).
OUTPUT. One of the ways to obtain oxygen in the laboratory is the decomposition of KMnO 4 . Oxygen is a colorless and odorless gas, 1.103 times heavier than air ( M r(O 2) \u003d 32, M r(air) \u003d 29, from which follows 32/29 1.103), slightly soluble in water. It reacts with simple substances, forming oxides.
Put the workplace in order (3 min): disassemble the appliance, arrange the dishes and accessories in their places.
Submit your notebooks for review.
Homework.
A task. Determine which of the iron compounds - Fe 2 O 3 or Fe 3 O 4 - is richer in iron?
| Given: | To find: |
| Fe 2 O 3, Fe 3 O 4 . |
(Fe) in Fe 2 O 3, "(Fe) to Fe 3 O 4 |
Solution
(X) = n A r(X)/ M r, where n- the number of atoms of the element X in the formula of the substance.
M r(Fe 2 O 3) \u003d 56 2 + 16 3 \u003d 160,
(Fe) \u003d 56 2/160 \u003d 0.7,
(Fe) = 70%,
M r(Fe 3 O 4) \u003d 56 3 + 16 4 \u003d 232,
"(Fe) \u003d 56 3/232 \u003d 0.724,
"(Fe) = 72.4%.
Answer. Fe 3 O 4 is richer in iron than Fe 2 O 3 .
During practical work, the teacher monitors the correctness of the performance of techniques and operations by students and notes in the skill record card (Table 2).
table 2
Skill record card
| Operations of practical work | Surnames of students | |||||
|---|---|---|---|---|---|---|
| BUT | B | IN | G | D | E | |
| Assembly of the device for obtaining oxygen | ||||||
| Checking the device for leaks | ||||||
| Fixing the test tube in the leg of the tripod | ||||||
| Alcohol lamp handling | ||||||
| Heating a test tube with KMnO 4 | ||||||
| Checking the release of O 2 | ||||||
| Collecting O 2 in a vessel by two methods: air displacement, water displacement |
||||||
| coal burning | ||||||
| Combustion of Fe (steel wire) | ||||||
| Experimental culture | ||||||
| Making work in a notebook | ||||||
Sample report on the practical work done (Table 1)
smoldering splinter
(charcoal) lights up brightly
in O 2
physical properties of O 2. Collecting O 2 by two methods:
air displacement (a),
water displacement (b)

slightly heavier than air, so
it is collected in a vessel placed on the bottom. Oxygen is slightly soluble in water


Lime water becomes cloudy, because a water-insoluble precipitate of CaCO 3 is formed:
CO 2 + Ca (OH) 2 CaCO 3 + H 2 O. Iron burns with a bright flame in oxygen:

with simple
substances - metals and non-metals. The formation of a white precipitate confirms the presence of CO 2 in the flask