Experimental substantiation of the basic principles of molecular kinetics. Basic provisions of the ICT and their experimental justification
12. Basic principles of molecular kinetic theory and their experimental substantiation. Mass and size of molecules
A theory that explains the structure and properties of bodies on the basis of the laws of motion and interaction of the particles that make up the bodies is called molecular kinetic.
The basic principles of molecular kinetic theory (MKT) are formulated as follows:
Any substance has a discrete (discontinuous) structure. It consists of individual particles (molecules, atoms, ions) separated by spaces.
Particles are in a state of continuous chaotic motion called thermal motion.
Particles interact with each other. In the process of their interaction, forces of attraction and repulsion arise.
The validity of ICT is confirmed by numerous observations and facts.
The presence of permeability, compressibility and solubility in substances indicates that they are not continuous, but consist of individual particles separated by spaces. Using modern research methods (electron and ion microscopes), images of the largest molecules were obtained.
Brownian motion and diffusion indicate that particles are in continuous motion.
The presence of strength and elasticity of bodies, the phenomenon of wetting, surface tension in liquids, etc. prove the existence of interaction forces between molecules.
Mass and size of molecules.
The size of molecules is a relative value. It is assessed as follows. Between molecules, along with attractive forces, repulsive forces also act, so molecules can only approach each other to a certain distance. The distance of maximum approach between the centers of molecules is called effective diameter of the molecule.(In this case, it is conventionally assumed that the molecules have a spherical shape.)
Using numerous methods for determining the masses and sizes of molecules, it has been established that, with the exception of molecules of organic substances containing a very large number of atoms, most molecules, in order of magnitude, have a diameter of 1·10 - 10 m and a mass of 1·10 - 26 kg.
Relative molecular weight.
Relative molecular (or atomic) mass M r (or A r ) they call a value equal to the ratio of the mass of a molecule (or atom) m o of this substance to 1/12 of the mass of a carbon atom m o C, i.e.
Relative molecular (atomic) mass is a dimensionless quantity.
Amount of substance. Molar mass. Molecule mass.
The amount of substance ν is a value equal to the ratio of the number of molecules (or atoms) N in a given body to the number of atoms N A in 0.012 kg of carbon, i.e. ν = N/ N A (N A is Avogadro’s number).
The molar mass M of a substance is the mass of 1 mole of this substance.
Consequently, the mass of a molecule (atom) can be determined from the relation
13. Ideal gas. Basic equation μmt of an ideal gas
An ideal gas is such a gas, when describing the properties of which the following assumptions are made: they do not take into account the intrinsic size of gas molecules and do not take into account the forces of interaction between them.
Thus, the model of an ideal gas is a set of chaotically moving material points that interact with each other and with the walls of a container containing gas only in direct collision.
Definition 1
Molecular kinetic theory is the doctrine of the structure and properties of matter, based on the idea of the existence of atoms and molecules, as the smallest particles of chemical substances.
Basic principles of the molecular kinetic theory of a molecule:
- All substances can be in liquid, solid and gaseous states. They are formed from particles that are made up of atoms. Elementary molecules can have a complex structure, that is, they can contain several atoms. Molecules and atoms are electrically neutral particles that, under certain conditions, acquire an additional electrical charge and become positive or negative ions.
- Atoms and molecules move continuously.
- Particles with an electrical nature of force interact with each other.
The main provisions of the ICT and their examples were listed above. There is little gravitational influence between the particles.
Figure 3. 1 . 1 . Trajectory of a Brownian particle.
Definition 2
The Brownian motion of molecules and atoms confirms the existence of the basic principles of molecular kinetic theory and experimentally substantiates it. This thermal movement of particles occurs with molecules suspended in a liquid or gas.
Experimental substantiation of the main provisions of the molecular kinetic theory
In 1827, R. Brown discovered this movement, which was caused by random impacts and movements of molecules. Since the process occurred chaotically, the blows could not balance each other. Hence the conclusion is that the speed of a Brownian particle cannot be constant, it is constantly changing, and the directional movement is depicted in the form of a zigzag, shown in Figure 3. 1 . 1 .
A. Einstein spoke about Brownian motion in 1905. His theory was confirmed in the experiments of J. Perrin in 1908 - 1911.
Definition 3
Corollary of Einstein's theory: offset square< r 2 >Brownian particle relative to the initial position, averaged over many Brownian particles, is proportional to the observation time t.
Expression< r 2 >= D t explains the diffusion law. According to theory, we have that D increases monotonically with increasing temperature. Random movement is visible in the presence of diffusion.
Definition 4
Diffusion- this is the definition of the phenomenon of penetration of two or more contacting substances into each other.
This process occurs quickly in a heterogeneous gas. Thanks to examples of diffusion with different densities, a homogeneous mixture can be obtained. When oxygen O2 and hydrogen H2 are in the same vessel with a partition, when it is removed, the gases begin to mix, forming a dangerous mixture. The process is possible when hydrogen is at the top and oxygen is at the bottom.
Interpenetration processes also occur in liquids, but much slower. If we dissolve a solid, sugar, in water, we obtain a homogeneous solution, which is a clear example of diffusion processes in liquids. Under real conditions, mixing in liquids and gases is masked by rapid mixing processes, for example, when convection currents occur.
The diffusion of solids is characterized by its slow speed. If the surface of interaction between metals is cleaned, you can see that over a long period of time atoms of another metal will appear in each of them.
Definition 5
Diffusion and Brownian motion are considered related phenomena.
When particles of both substances interpenetrate, the movement is random, that is, chaotic thermal movement of molecules is observed.
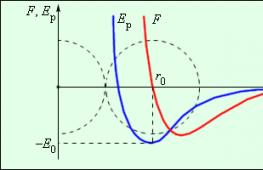
The forces acting between two molecules depend on the distance between them. Molecules contain positive and negative charges. At large distances, the forces of intermolecular attraction predominate; at small distances, the forces of repulsion predominate.
Drawing 3 . 1 . 2 shows the dependence of the resulting force F and potential energy E p of interaction between molecules on the distance between their centers. At a distance r = r 0, the interaction force becomes zero. This distance is conventionally taken as the diameter of the molecule. At r = r 0, the potential energy of interaction is minimal.
Definition 6
To move two molecules apart with a distance r 0, you should communicate E 0, called binding energy or potential well depth.

Figure 3. 1 . 2.The power of interaction F and potential energy of interaction E r two molecules. F > 0– repulsive force, F< 0 - force of gravity.
Since molecules are small in size, simple monatomic ones can be no more than 10 - 10 m. Complex ones can reach sizes hundreds of times larger.
Definition 7
The random chaotic movement of molecules is called thermal movement.
As temperature increases, the kinetic energy of thermal motion increases. At low temperatures, the average kinetic energy, in most cases, turns out to be less than the depth of the potential well E 0 . This case shows that molecules flow into a liquid or solid with an average distance between them r 0 . If the temperature rises, then the average kinetic energy of the molecule exceeds E 0, then they fly apart and form a gaseous substance.
In solids, molecules move randomly around fixed centers, that is, equilibrium positions. They can be distributed in space in an irregular manner (in amorphous bodies) or with the formation of ordered volumetric structures (crystalline bodies).
Aggregate states of substances
The freedom of thermal movement of molecules is visible in liquids, since they are not tied to centers, which allows movements throughout the entire volume. This explains its fluidity.
Definition 8
If the molecules are located closely, they can form ordered structures with several molecules. This phenomenon is called short-range order. Long range order characteristic of crystalline bodies.
The distance between molecules in gases is much greater, so the acting forces are small, and their movements proceed along a straight line, awaiting the next collision. The value of 10 – 8 m is the average distance between air molecules under normal conditions. Since the interaction of forces is weak, the gases expand and can fill any volume of the vessel. When their interaction tends to zero, they speak of an ideal gas.
Kinetic model of an ideal gas
In μt, the amount of substance is considered proportional to the number of particles.
Definition 9
Mole- this is the amount of substance containing as many particles (molecules) as there are atoms in 0.012 kg of carbon C 12. A carbon molecule consists of one atom. It follows that 1 mole of a substance has the same number of molecules. This number is called constant Avogadro N A: N A = 6.02 ċ 1023 mol – 1.
Formula for determining the amount of a substance ν is written as the ratio N of the number of particles to Avogadro’s constant N A: ν = N N A .
Definition 10
Mass of one mole of substance is called the molar mass M. It is fixed in the form of the formula M = N A ċ m 0.
The expression of molar mass is made in kilograms per mole (kg/mol).
Definition 11
If a substance contains one atom, then we can talk about the atomic mass of the particle. A unit of an atom is 1 12 masses of the carbon isotope C 12, called atomic mass unit and is written as ( A. eat.): 1 a. e.m. = 1.66 ċ 10 – 27 kg.
This value coincides with the mass of the proton and neutron.
Definition 12
The ratio of the mass of an atom or molecule of a given substance to 1 12 mass of a carbon atom is called relative mass.
If you notice an error in the text, please highlight it and press Ctrl+Enter
Molecular kinetic theory is a branch of physics that studies the properties of various states of matter, based on the idea of the existence of molecules and atoms as the smallest particles of matter. ICT is based on three main principles:
1. All substances consist of tiny particles: molecules, atoms or ions.
2. These particles are in continuous chaotic motion, the speed of which determines the temperature of the substance.
3. Between particles there are forces of attraction and repulsion, the nature of which depends on the distance between them.
The main provisions of the ICT are confirmed by many experimental facts. The existence of molecules, atoms and ions has been proven experimentally, the molecules have been sufficiently studied and even photographed using electron microscopes. The ability of gases to expand indefinitely and occupy the entire volume provided to them is explained by the continuous chaotic movement of molecules. The elasticity of gases, solids and liquids, the ability of liquids to wet some solids, the processes of coloring, gluing, retention of shape by solids and much more indicate the existence of forces of attraction and repulsion between molecules. The phenomenon of diffusion - the ability of molecules of one substance to penetrate into the spaces between the molecules of another - also confirms the main provisions of MCT. The phenomenon of diffusion explains, for example, the spread of odors, the mixing of dissimilar liquids, the process of dissolving solids in liquids, and the welding of metals by melting them or by pressure. Confirmation of the continuous chaotic movement of molecules is also Brownian motion - the continuous chaotic movement of microscopic particles insoluble in liquid.
The motion of Brownian particles is explained by the chaotic motion of liquid particles that collide with microscopic particles and set them in motion. It has been experimentally proven that the speed of Brownian particles depends on the temperature of the liquid. The theory of Brownian motion was developed by A. Einstein. The laws of particle motion are statistical and probabilistic in nature. There is only one known way to reduce the intensity of Brownian motion - decreasing the temperature. The existence of Brownian motion convincingly confirms the movement of molecules.
Any substance consists of particles, therefore the amount of substance v is considered to be proportional to the number of particles, i.e., structural elements contained in the body.
The unit of quantity of a substance is the mole. A mole is the amount of a substance containing the same number of structural elements of any substance as there are atoms in 12 g of C12 carbon. The ratio of the number of molecules of a substance to the amount of substance is called Avogadro's constant:
Avogadro's constant shows how many atoms and molecules are contained in one mole of a substance. Molar mass is the mass of one mole of a substance, equal to the ratio of the mass of the substance to the amount of the substance:
Molar mass is expressed in kg/mol. Knowing the molar mass, you can calculate the mass of one molecule:
The average mass of molecules is usually determined by chemical methods; Avogadro's constant is determined with high accuracy by several physical methods. The masses of molecules and atoms are determined with a significant degree of accuracy using a mass spectrograph.
The masses of molecules are very small. For example, the mass of a water molecule:
Molar mass is related to the relative molecular mass of Mg. Relative molecular weight is a value equal to the ratio of the mass of a molecule of a given substance to 1/12 of the mass of a C12 carbon atom. If the chemical formula of a substance is known, then using the periodic table its relative mass can be determined, which, when expressed in kilograms, shows the molar mass of this substance.
Molecular kinetic theory is a branch of physics that studies the properties of various states of matter, based on the idea of the existence of molecules and atoms as the smallest particles of matter. ICT is based on three main principles:
1. All substances consist of tiny particles: molecules, atoms or ions.
2. These particles are in continuous chaotic motion, the speed of which determines the temperature of the substance.
3. Between particles there are forces of attraction and repulsion, the nature of which depends on the distance between them.
The main provisions of the ICT are confirmed by many experimental facts. The existence of molecules, atoms and ions has been proven experimentally, the molecules have been sufficiently studied and even photographed using electron microscopes. The ability of gases to expand and occupy indefinitely all the volume provided by it is explained by the continuous chaotic movement of molecules. Elasticity gases, solids and liquids, ability of liquids
moistening some solids, the processes of coloring, gluing, maintaining shape by solids and much more indicate the existence of forces of attraction and repulsion between molecules. The phenomenon of diffusion - the ability of molecules of one substance to penetrate into the spaces between the molecules of another - also confirms the main provisions of MCT. The phenomenon of diffusion explains, for example, the spread of odors, the mixing of dissimilar liquids, the process of dissolving solids in liquids, and the welding of metals by melting them or by pressure. Confirmation of the continuous chaotic movement of molecules is also Brownian motion - the continuous chaotic movement of microscopic particles insoluble in liquid.
The motion of Brownian particles is explained by the chaotic motion of liquid particles that collide with microscopic particles and set them in motion. It has been experimentally proven that the speed of Brownian particles depends on the temperature of the liquid. The theory of Brownian motion was developed by A. Einstein. The laws of particle motion are statistical and probabilistic in nature. There is only one known way to reduce the intensity of Brownian motion - decreasing the temperature. The existence of Brownian motion convincingly confirms the movement of molecules.
Any substance consists of particles, therefore amount of substance is considered to be proportional to the number of particles, i.e., structural elements contained in the body, v.
The unit of quantity of a substance is mole.Mole- this is the amount of substance containing the same number of structural elements of any substance as there are atoms in 12 g of carbon C 12. The ratio of the number of molecules of a substance to the amount of substance is called Avogadro's constant:
n a= N/ v. na = 6,02 10 23 mole -1 .
Avogadro's constant shows how many atoms and molecules are contained in one mole of a substance. Molar mass is a quantity equal to the ratio of the mass of a substance to the amount of substance:
M = m/ v.
Molar mass is expressed in kg/mol. Knowing the molar mass, you can calculate the mass of one molecule:
m 0 = m/N = m/vN A= M/ N A
The average mass of molecules is usually determined by chemical methods; Avogadro's constant is determined with high accuracy by several physical methods. The masses of molecules and atoms are determined with a significant degree of accuracy using a mass spectrograph.
The masses of molecules are very small. For example, the mass of a water molecule: t = 29.9 10 -27 kg.
Molar mass is related to the relative molecular mass of Mr. Relative molar mass is a value equal to the ratio of the mass of a molecule of a given substance to 1/12 of the mass of the C 12 carbon atom. If the chemical formula of a substance is known, then using the periodic table its relative mass can be determined, which, when expressed in kilograms, shows the molar mass of this substance.